DIAGNOSE AND START THE JOURNEY TO HELP CURE CHRONIC HCV1
>4 million
people living in United States with HCV, only 5% treated2,3,a
aBased on NHANES+ model estimates of current HCV infections from 2017-2020 and AASLD treatment numbers from 2020.2,3
1 in 2 people
who inject drugs in the United States have HCV4,b
b45% of people 17-35 years of age.4
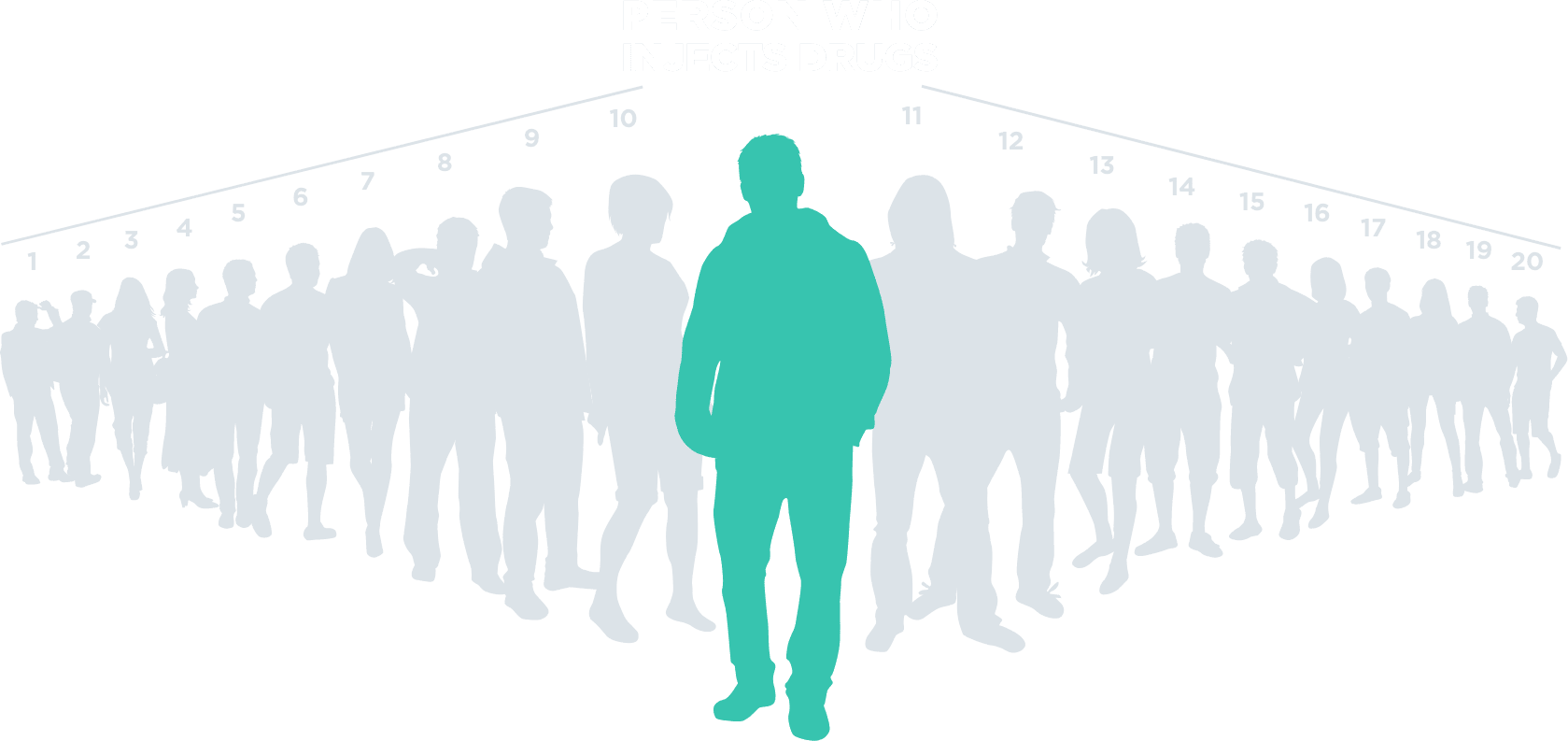
Within the first 3 years of initial infection
Each person with HCV who injects drugs is likely to infect ~20 people5,c

cBased on the 2021 NIH National Institute on Drug Abuse Heroin Research Project.5
GUIDELINES RECOMMEND UNIVERSAL TESTING AND TREATMENT FOR HEPATITIS C, INCLUDING IN PEOPLE WHO INJECT DRUGS6-8
|
Test All Adult Patients At Least Once |
Test All At-Risk Patients |
Begin Treating All Diagnosed Patients |
|
|---|---|---|---|
| AASLD/IDSA |
|
|
|
| CDC |
|
|
|
| USPSTF |
|
|
|
d Unless they have limited life expectancy that cannot be remediated by HCV treatment, liver transplantation, or another directed therapy.6
e Except in settings where the prevalence of HCV infection (HCV RNA-positivity) is <0.1%.7
f Patients need further evaluation before treatment.7
g Recommendations for screening of adults (18-79 years).8
h Adults with a positive result are usually followed up with a diagnostic evaluation8