INDICATION
EPCLUSA (sofosbuvir 400 mg/velpatasvir 100 mg, 200 mg/50 mg tablets; 200 mg/50 mg, 150 mg/37.5 mg oral pellets) is indicated for the treatment of patients 3 years of age and older with chronic hepatitis C virus (HCV) genotype 1-6 infection without cirrhosis or with compensated cirrhosis and in combination with ribavirin for those with decompensated cirrhosis.
Please see below for Important Safety Information for EPCLUSA.
Access & Support
FEEL CONFIDENT IN YOUR HCV PATIENTS’ ACCESS TO TREATMENT
Featured patient
compensated by Gilead.
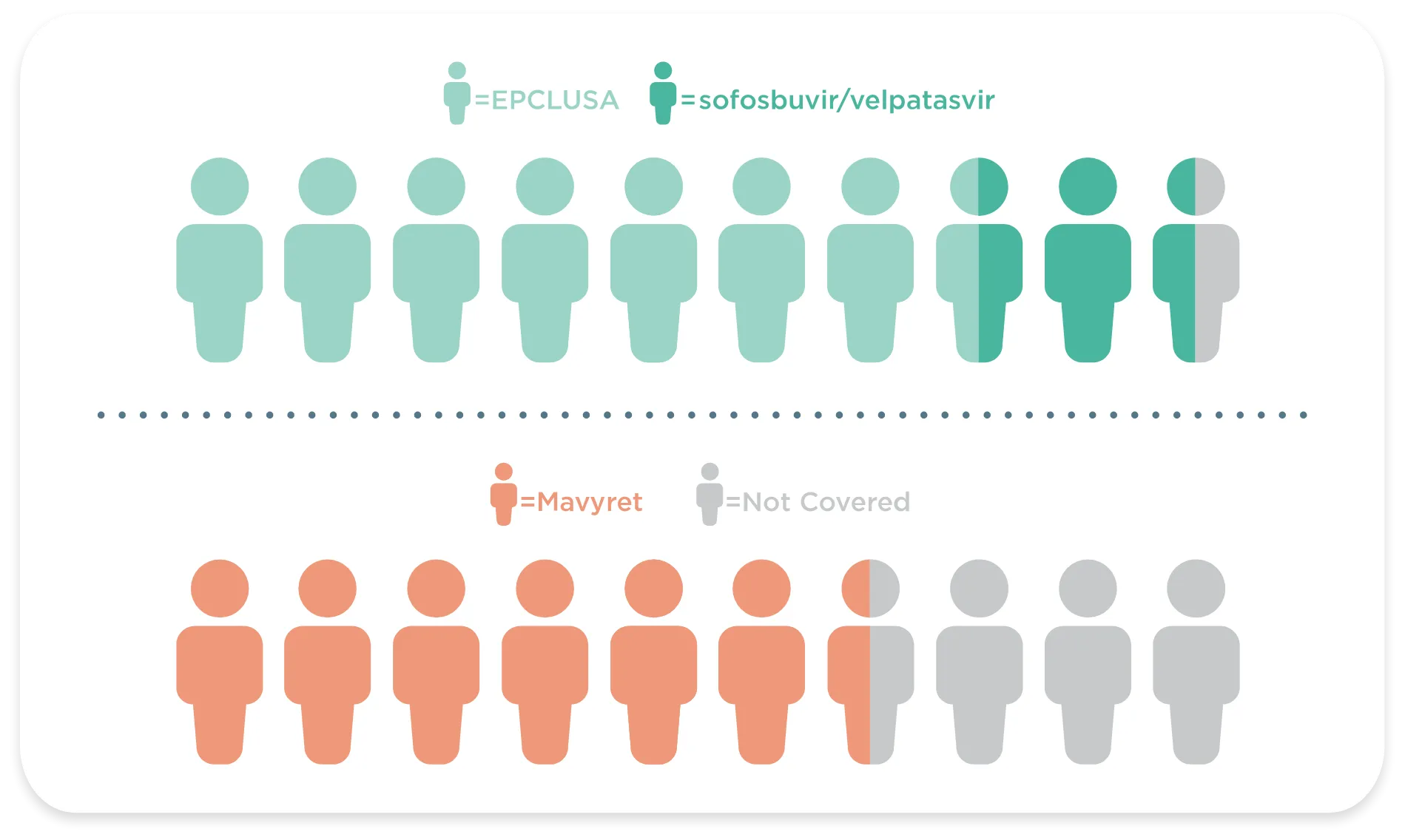
Only EPCLUSA and sofosbuvir/velpatasvir, its Authorized Generic, provide 94% of all patients access to treatment nationally (vs 68% for Mavyret®)1
Placement on the formulary is not intended to imply any claims regarding safety, efficacy, or comparability of products.
Based on national covered (Exclusive or Preferred) lives as of November 2023. Local coverage may differ.
Exclusive = is on formulary while other DAAs are not, has a lower tier status than other DAAs, or is required in a step therapy process vs other DAAs.
Preferred = shares the lowest tier status with at least one DAA.
COMMERCIAL
Cost should not be a barrier to treatment. Commercial patients may pay as little as $15 for a full course of treatment of EPCLUSA or its Authorized Generic.2
$5 Co-Pay Coupon

Help patients register for a co-pay coupon. Eligible commercially insured individuals enrolled in the Gilead Support Path co-pay coupon program may pay as little as $15 for a full course of EPCLUSA or sofosbuvir/velpatasvir. Restrictions apply. Subject to change.2
See the link below for full terms and conditions
Visit MySupportPath.comVisit the EPCLUSA patient website to learn more
Check Your Patients' EligibilityCommercial patients may pay as little as $5 per co-pay for their medicine. Patients are not eligible if they are enrolled in a government healthcare prescription drug program such as Medicare Part D or Medicaid.
The EPCLUSA Co-pay Coupon Program will cover the out-of-pocket costs for EPCLUSA prescriptions up to a maximum of 25% of the catalog price of a 12-week regimen of EPCLUSA.

Commercial patients may pay as little as $5 per co-pay for their medicine. Patients are not eligible if they are enrolled in a government healthcare prescription drug program such as Medicare Part D or Medicaid.
The EPCLUSA Co-pay Coupon Program will cover the out-of-pocket costs for EPCLUSA prescriptions up to a maximum of 25% of the catalog price of a 12-week regimen of EPCLUSA.
MEDICARE
Approximately 8 out of 10 Medicare patients taking EPCLUSA or its Authorized Generic pay $15 or less for the full course of treatment.2,a
For your patients with insurance or who are uninsured, our team of Support Path Program Specialists can provide information on coverage and financial support options that help get patients started with treatment once mdication has been prescribed.
Talk with Support Path:
aGilead Analysis of patient data provided by Symphony Health (Patient Transaction Data for HCV market) Jul '21-Aug '22, accessed Aug '22. Out-of-pocket costs are based on average cost sharing on claims for new and continuing patients who take EPCLUSA, or the Authorized Generic of EPCLUSA, after any secondary benefit is applied.2
With EPCLUSA and its Authorized Generic, you have access for the majority of your insured HCV patients.
When you write a prescription for EPCLUSA, the pharmacy may fill for either EPCLUSA
or its Authorized Generic, depending on the patient’s coverage.
![]()
The Authorized Generic of EPCLUSA has the same clinical profile as branded EPCLUSA.
If the Authorized Generic of EPCLUSA is covered by your patient's formulary, it will be automatically substituted if you have written the prescription for EPCLUSA in states with mandatory generic substitution laws. In states with permissive substitution laws, pharmacies will substitute the Authorized Generic per instruction from payers.
You can also write the Authorized Generic name (ie, sofosbuvir/velpatasvir) directly.
Support for your patients through their treatment journey
NURSES & EPCLUSA EDUCATORS
- Nurses and EPCLUSA Educators are available through phone, text, or email, 7 days a week, to answer questions your patients might have about hepatitis C or EPCLUSA
- Nurses and EPCLUSA Educators can provide ongoing contact with your patients to better understand their concerns and provide specific resources they need every step of the way
Nurse support does not take the place of talking with a physician.
EPCLUSA in-office brochure
A resource you can share with your patients highlighting HCV education, financial support options, and patient support
DownloadEPCLUSA brochure (en Español)
A resource in Spanish to share with your patients highlighting HCV education, financial support options, and patient support
DownloadChris’ Hep C story brochure
A resource you can share with your patients about Chris’ struggle given his history of addiction, and how EPCLUSA worked for his Hep C
DownloadHave a patient starting treatment? Support Path® is here to help
Our team of Support Path Program Specialists can provide information on coverage and financial support options that help get patients started with treatment once medication has been prescribed

Talk with Support Path:
Visit MySupportPath.com


